Abstract
BACKGROUND: Mantle cell lymphoma (MCL) is a B-cell non-Hodgkin lymphoma with variable clinical outcomes. Commonly used risk stratification tools (Ki67 IHC, MIPI) in newly diagnosed MCL are not frequently used when selecting therapy, resulting in treatment choice being dictated by age and co-morbidities rather than disease biology. The MCL35 risk score was developed as a more reliable measure of proliferation and has been shown to be prognostic and can risk stratify younger transplant eligible MCL patients into three groups with significantly different overall survival (OS; Scott et al. 2017; Holte et al. 2018) but has not been evaluated in older transplant ineligible patients. We report results evaluating the prognostic value of the MCL35 assay in older MCL patients (≥65) treated with frontline bendamustine/rituximab (BR).
METHODS: Archived tissue samples from 119 patients age ≥65 years treated with BR from collaborating Lymphoma/Leukemia Molecular Profiling Project (LLMPP) sites and the LEO/MER cohort were collected and analyzed using the MCL35 assay and stratified into three distinct risk groups (low, standard, and high risk). Association between MCL35 proliferation scores and OS were estimated by the Kaplan-Meier method and hazard ratios were calculated. Associations between Ki67, s-MIPI, p53 IHC status, morphology and OS were also evaluated.
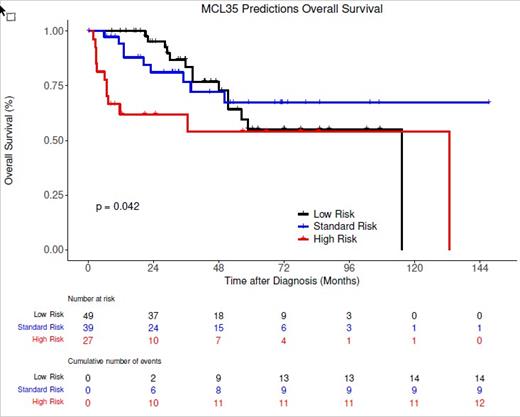
RESULTS: The MCL35 assay was run on tissue samples from 119 patients. Median patient age was 74 (range 65-93) and 69.5% were male. Ki67 was <30% in 29 patients (24%) and ≥30% in 90 patients (76%). Simplified MIPI (s-MIPI) score was 0-3 in 21 patients (24%), 4-5 in 42 patients (48%) and ≥6 in 25 patients (28%). Thirty-one did not have sufficient data to calculate a s-MIPI score. MCL35 was low risk in 51 patients (43%), standard risk in 39 patients (33%) and high risk in 29 patients (24%). Eleven patients had blastic morphology, 7 had pleomorphic morphology and the remainder were classic morphology (n=56). Of 57 samples with p53 IHC staining 7 (12.3%) were positive. At a median follow up of 33.4 months, 82 patients were alive and 35 had died. Patients with high risk MCL35 score had inferior OS compared to low risk (HR 2.27, 95% CI: 1.03-5.00; p=0.042) while standard risk was not statistically significant compared to low risk (HR 0.87, 95% CI: 0.37-2.0; p=0.740)(Figure 1). Ki67 IHC using a cutoff of ≥ 30% and 10%-29% was not significantly associated with OS compared to Ki67 <10% ( Ki67 ≥ 30% vs. Ki67 < 10%, HR 0.87, 95% CI: 0.12-6.41; p=0.892, Ki67 ≥ 10%-29% vs. Ki67 < 10%, HR 0.32, 95% CI: 0.04-2.83; p=0.303), however high s-MIPI score (≥6) (s-MIPI ≥6 vs. s-MIPI 0-3, HR 3.86, 95% CI 1.20-12.5; p=0.024) and positive p53 IHC (HR: 9.51, 3.26-27.7; p <0.001) were both associated with poor OS. Eighteen cases were blastic/pleomorphic by morphology, 12 of which were in the high-risk group by MCL35, and this subset also had worse survival than classic MCL (p=0.0052).
CONCLUSIONS: These results suggest high risk MCL35 score is a prognostic biomarker of poor OS in patients >65 with MCL treated with BR. Conversely, Ki67 was not significantly associated with OS in these patients. Additional clinical validation using a larger sample size from the E1411 study is planned. If similar results are found, the MCL35 assay in combination with s-MIPI and p53 status may have utility in stratifying patients into risk adapted treatment arms in future prospective clinical trial designs.
Maurer: BMS: Research Funding; Genentech: Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Nanostring: Research Funding. Villa: Janssen: Honoraria; Gilead: Honoraria; AstraZeneca: Honoraria; AbbVie: Honoraria; Seattle Genetics: Honoraria; Celgene: Honoraria; Lundbeck: Honoraria; Roche: Honoraria; NanoString Technologies: Honoraria. Habermann: Seagen: Other: Data Monitoring Committee; Incyte: Other: Scientific Advisory Board; Tess Therapeutics: Other: Data Monitoring Committee; Morphosys: Other: Scientific Advisory Board; Loxo Oncology: Other: Scientific Advisory Board; Eli Lilly & Co.,: Other: Scientific Advisor. Cohen: Janssen, Adicet, Astra Zeneca, Genentech, Aptitude Health, Cellectar, Kite/Gilead, Loxo, BeiGene, Adaptive: Consultancy; Genentech, BMS/Celgene, LAM, BioINvent, LOXO, Astra Zeneca, Novartis, M2Gen, Takeda: Research Funding. Hill: Celgene (BMS): Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria; Gentenech: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; AstraZenica: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; Incyte/Morphysis: Consultancy, Honoraria, Research Funding. Raess: Scopio Labs: Research Funding. Scott: Celgene: Consultancy; NanoString Technologies: Patents & Royalties: Patent describing measuring the proliferation signature in MCL using gene expression profiling.; BC Cancer: Patents & Royalties: Patent describing assigning DLBCL COO by gene expression profiling--licensed to NanoString Technologies. Patent describing measuring the proliferation signature in MCL using gene expression profiling. ; Rich/Genentech: Research Funding; Janssen: Consultancy, Research Funding; Incyte: Consultancy; Abbvie: Consultancy; AstraZeneca: Consultancy. Rimsza: NanoString Technologies: Other: Fee-for-service contract.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal